Department of Molecular biophysics
(Created page with "=='''About'''== Laboratory of Molecular Biophysics has beenestablished in April 2012 based on the research group headed by Dr. P. Belan at the Department of General Physiology...") |
(→About) |
||
| Line 1: | Line 1: | ||
=='''About'''== | =='''About'''== | ||
| − | Laboratory of Molecular Biophysics has beenestablished in April 2012 based on the research group headed by Dr. P. Belan at the Department of General Physiology of the Nervous System. Mechanisms regulating intracellular cytoplasmic Ca2+ concentration and Ca2+ signalling in different types of excitable cells are the main subject of our research during recent years. Acutely isolated and primary cultured neurons as well as slices and ex vivo intact spinal cord preparationhave been used in the experiments. Mainly, optical (digital imaging and confocal microscopy) and conventional electrophysiological methods (microelectrodes, iontophoresis, patch clamp) had been employed in our studies. | + | Laboratory of Molecular Biophysics has beenestablished in April 2012 based on the research group headed by [[P.V._Belan|Dr. P. Belan]] at the Department of General Physiology of the Nervous System. Mechanisms regulating intracellular cytoplasmic Ca2+ concentration and Ca2+ signalling in different types of excitable cells are the main subject of our research during recent years. Acutely isolated and primary cultured neurons as well as slices and ex vivo intact spinal cord preparationhave been used in the experiments. Mainly, optical (digital imaging and confocal microscopy) and conventional electrophysiological methods (microelectrodes, iontophoresis, patch clamp) had been employed in our studies. |
| − | + | ||
=='''Research'''== | =='''Research'''== | ||
Revision as of 16:16, 22 October 2015
Contents |
About
Laboratory of Molecular Biophysics has beenestablished in April 2012 based on the research group headed by Dr. P. Belan at the Department of General Physiology of the Nervous System. Mechanisms regulating intracellular cytoplasmic Ca2+ concentration and Ca2+ signalling in different types of excitable cells are the main subject of our research during recent years. Acutely isolated and primary cultured neurons as well as slices and ex vivo intact spinal cord preparationhave been used in the experiments. Mainly, optical (digital imaging and confocal microscopy) and conventional electrophysiological methods (microelectrodes, iontophoresis, patch clamp) had been employed in our studies.
Research
Signaling of neuronal Ca2+ sensor proteins
Decoding glutamate receptor activation by the Ca2+sensor proteins
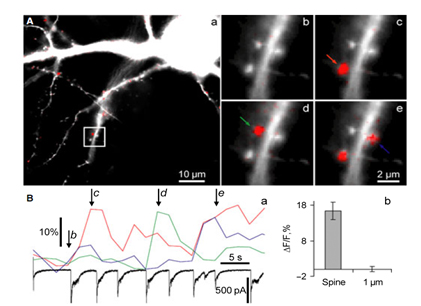
We study biophysical and physiological mechanisms of NCS (Neuronal Calcium Sensor) protein signaling in hippocampal neurons. Decoding of complex spatio-temporal patterns of [Ca2+]i changes by a Ca2+sensor proteins, hippocalcin and neurocalcin δ, and involvement of Ca2+-dependent protein translocation in neuronal signal transduction is in a center of these studies. The most important achievement in this line of researchisclear understanding that hippocalcinand neurocalcin δmay differentially decode various spatiotemporal patterns of glutamate receptor activation into site- and time-specific translocation to their membranous targets. Hippocalcin also possesses an ability to produce local signalling at the single synaptic level providing a molecular mechanism for homosynaptic plasticity.Examples of spontaneous hippocalcin-YFP translocation to a set of spines correlated with bursts of postsynaptic currents are shown in the Figure 1.
Figure 1. Strong activation of synaptic NMDARs induced hippocalcin-YFP translocation to dendritic spines. (A) An overlay of morphological (white) and hippocalcin-YFP translocation (red) images of neuron during a spontaneous burst of synaptic NMDAR-dependent currents at the time indicated as d in Ba. All synapses that were active during the burst appear in red. Panels b–e demonstrate overlays of morphological (white) and translocation images taken at the times indicated by respective letters in italic in Ba. Colour arrows indicate spines for which time courses of hippocalcin-YFP translocation are demonstrated in Ba.NMDAR-dependent currents were simultaneously recorded in whole-cell voltage clamp mode (holding potential )70 mV) and shown in Ba (black trace). (Bb) Values of hippocalcin-YFP translocation to spines compared with those in a dendritic tree at 1 lm from the respective spines.
Analysis of the mechanisms underlying GABAergic synaptic transmission
Short-term plasticity of GABAergic synaptic transmission in the hippocampus
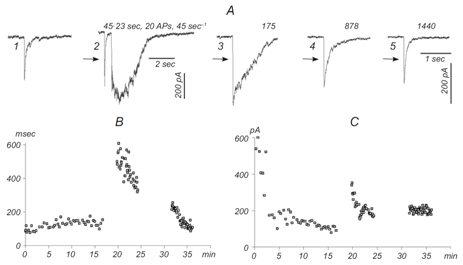
This part of our research is devoted to studies of different synaptic mechanisms contributing to GABAergic synaptic transmission in the hippocampus. Mechanisms of short-term depression and non-phasic vesicular release are in focus of our current research(A. R. Stepanyuk, Borisyuk, Tsugorka, & Belan, 2014)(Borisyuk, Stepanyuk, & Belan, 2014). We have recently found a new type of short-term plasticity in cultured hippocampal neuronsmanifested as activity-dependent potentiation of an asynchronous component of GABAergic synaptic currents,аIPSCs (Fig. 2)(Borisyuk et al., 2014).
Figure 2. Potentiation of an asynchronous component of inhibitory postsynaptic current (IPSC).A) Slowdown of the decay kinetics and potentiation of the asynchronous component of evoked IPSC (eIPSC).Dual patch clamp recordings were performed in a pair of synapticallyconnected cultured hippocampal neurons. 1) eIPSC in the control, 2) eIPSC developing after stimulation of the presynaptic cell (20 stimuli at 45 sec–1); 3-5) successive recordings of eIPSCs at different times after cessation of stimulation (time intervals in sec are shown at the right above). B and C) Time-dependence of eIPSC decay (B,msec) and amplitude (C, pA).
Simultaneously with potentiation of аIPSC, we observed plateau-like inward currentsin the presynaptic neuron. The charge transferred by this current correlated significantly with the decay time of evoked IPSC in the postsynaptic neuron (mean correlation coefficient 0.83±0.10)suggesting that the inward current mediates аIPSCpotentiation. We hypothesize that theobserved plasticity may endogenously regulate the efficacy of GABAergic synaptic transmission in the hippocampus. In the ongoing research we are studying pre- and postsynaptic mechanisms involved in this new type of inhibitory synaptic plasticity.
Maximum likelihood estimation of biophysical parameters of synaptic receptors from macroscopic currents
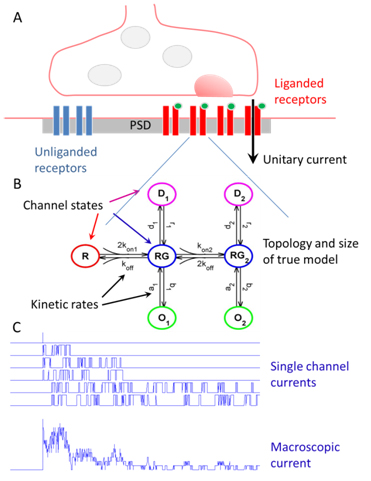
Dendritic integration and neuronal firing patterns strongly depend on biophysical properties of synaptic ligand-gated channels. However, precise estimation of biophysical parameters of these channels in their intrinsic environment is complicated and still unresolved problem. We have recently described novel methods based on a maximum likelihood approach that allows to estimate not only the unitary current of synaptic receptor channels but also their multiple conductance levels, kinetic constants, the number of receptors bound with a neurotransmitter, and the peak open probability from experimentally feasible number of postsynaptic currents (A. R. Stepanyuk, Borisyuk, & Belan, 2011, A. Stepanyuk, Borisyuk, & Belan, 2014). The new method also improves the accuracy of evaluation of unitary current as compared to the peak-scaled non-stationary fluctuation analysis, leading to a possibility to precisely estimate this important parameter from a few postsynaptic currents recorded in steady-state conditions. Estimation of unitary current with this method is robust even if postsynaptic currents are generated by receptors having different kinetic parameters, the case when peak-scaled non-stationary fluctuation analysis is not applicable. Thus, with the new method, routinely recorded postsynaptic currents could be used to study the properties of synaptic receptors in their native biochemical environment.
Figure 3.Schematic description of research. Synaptic receptors embedded in the postsynaptic density (PSD) (A) are not accessible for single channel patch clamp recordings (C). At the same time macroscopic currents elicited in response to presynaptic stimulation of the channels are a sum of single-channel currents (C) and can be easily recorded in a whole-configuration. The new suggested methods use all information included in the statistical properties of macroscopic current fluctuations (C) and given appropriately recorded and filtered currents they can reliably estimate kinetic rates of realistically complex models (B) with accuracy of the single-channel analysis. They can also estimate unitary current (A) and the number of liganded and unliganded receptors in the PSD (A). The new methodology that will be developed in the framework of the project will also allow evaluating the size and topology of synaptic channel model (B) together with the biophysical parameters mentioned above.
Further elaboration of this approach that we are doing now will lead to development of user-friendly software that may be widely used to quantitatively study interaction of synaptic channels with pharmacological agents and modulation of channel functioning in normal and pathological conditions.
Peripheral and central mechanisms of neuropathic pain
Role of T-type Ca2+ channels in central processes of nociceptive neurons in maintenance of diabetic neuropathy
Peripheral diabetic neuropathy (PDN), occurring in about 60% - 70% of diabetic patients, is among the most severe consequences of diabetes. Chronic pain characterized by spontaneous deep aching or burning pain, as well as by mechanical allodynia and thermal hyperalgesia is one of the most disturbing symptoms of PDN. However, the specific cellular mechanisms responsiblefor development and maintenance of peripheral diabetic neuropathy are mainly unknown. We have recently found that different types of nociceptive DRG neurons demonstrate diabetes-induced upregulation of CaV3.2 subtype of T-type Ca2+ channels during different stages of diabetes development(Khomula et al., 2013, Khomula et al., 2014, Duzhyy, Viatchenko-Karpinski, Khomula, Voitenko, & Belan, 2015). The upregulation of T-type channels resulted in the increased neuronal excitability of nociceptive neurons revealed by a lower threshold for action potential initiation, prominent afterdepolarizing potentials and burst firing. The upregulation T-type channels and increased excitability of neurons may contribute to thermal hyperalgesia in early diabetes and nonthermal nociception (e.g. mechanical allodynia and hyperalgesia and spontaneous pain) at its later-stage.We hypothesize that T-type Ca2+ channels are functionally expressed in central nociceptive processes and that upregulation of these channels under diabetic conditions amplifies peripheral action potentials in these neurons to busting discharges leading to changes in nociceptive output of lamina I projection neurons of the spinal cord and maintenance of painful diabetic neuropathy. Since direct electrophysiological measurements of T-type channel functioning in central axons of nociceptive DRG neurons are not methodologically feasible we are testing this hypothesis by an innovative combination of state-of-the-art approaches that include a novel whole spinal cord preparation that preserves the complex organization of dorsal roots in the spinal cord, Ca2+ imaging in identified central axons of specific types of primary nociceptive neurons using genetic Ca2+ indicators and profound computer modelling.
Peripheral and central mechanisms underlying inflammatory chronic pain
We have recently found that complete Freund adjuvant (CFA)-induced peripheral inflammation, a well-known model of chronic inflammatory pain, prominently augments excitatory neurotransmission in rat dorsal horn lamina II neurons exhibiting adapting firing patterns (Kopach, Krotov, Belan, & Voitenko, 2015) and apparently representing excitatory glutamatergic interneurons. At the same time this peripheral inflammation decreases excitatory drive to the tonic firing lamina II neurons most of which are inhibitory. The inhibitory drive is also increased to the inhibitory neurons and decreased to the excitatory ones as a result of the inflammation (Kopach et al., 2015). Thus, the balance between excitation and inhibition in the lamina II of dorsal horn is strongly shifted toward the excitation (Kopach et al., 2015). The lamina II interneurons directly synapse onto lamina I projection neurons (PNs), the main output of painful signaling in the spinal cord. Thus, these inflammatory-induced, neuron-type specific changes in synaptic activity in lamina II neurons most probably results in an increase of the excitatory drive and a decrease of the inhibitory drive to lamina I PNs. In its turn it may increase PN excitability, hence contributing to maintenance of the inflammatory pain. In this research we aim to test this hypothesis and to study what cellular and molecular mechanisms underlie increased excitability of PNs under inflammatory conditions.
Members
| Name | Work number | Mobile number | |
|---|---|---|---|
| Belan P.V. | pasha@biph.kiev.ua | 380 44 256 2053 | 380 50 353 2433 |
| Kononenko M.I. | |||
| Gryshchenko O.V. | |||
| Saftenku О.Е. | |||
| Stepanyuk A.R. | |||
| Cherkas V.P. | |||
| Dovgan O.V. | |||
| Borisyuk A.L. | |||
| Krotov V.V. | |||
| Sheremet E. | |||
| Dromaretskiy A.V | |||
| Bagatska O.V. | |||
| Edutenko M. | |||
| Bozhenko A. | |||
| Burdakova A. | |||
| Osipenko D. | |||
| Molodova O. |