Department of Cytology
(→Scientific advances. Selected publications for years 2011-2015) |
(→Research methods) |
||
| Line 53: | Line 53: | ||
=== Research methods === | === Research methods === | ||
| − | * modelling of neurodegeneration in vivo (cerebral ischemia, Parkinson’s disease, perinatal pathology of CNS | + | * modelling of neurodegeneration in vivo (cerebral ischemia, Parkinson’s disease, perinatal pathology of CNS, exocrine pancreatic insufficiency) and in vitro (cerebral ischemia, Alcheimer’s disease, stress); |
* microscopy: light, confocal, electron; | * microscopy: light, confocal, electron; | ||
* immunohystochemical methods; | * immunohystochemical methods; | ||
Revision as of 18:34, 20 April 2018
Cytology Department was founded in 1996 on the basis of laboratory of neurocytology. Honoured master of sciences and engineering of Ukraine, laureate of State Prize of Ukraine in sciences and engineering, Prof. Galyna Skibo is the unchallenged leader of the Department.Contents |
Science
The research activity of Department of Cytology is focused on the study of molecular and cellular mechanisms of nervous tissue damages related to the development of neurodegenerative diseases (cerebral ischemia, Alzheimer's disease, Parkinson's disease) or some pathological states (perinatal pathology of the central nervous system, stress, exocrine pancreatic failure) and the investigation of approaches for prevention and correction of neurodegeneration processes. The important part of the research activity is testing of pharmacological preparations to identify their potential neuroprotective properties. Recently experiments have been started aimed at studying of possible applications of cell therapy for the treatment of brain tissue lesions caused by cerebral ischemia. In two brain ischemia models (in vitro and in vivo) it was revealed that transient ischemia induced destructive changes in neurons and remodeling of synaptic apparatus in CA1 area of hippocampus – the brain structure responsible for the formation of memory and learning.
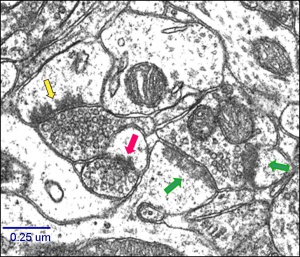
The temporal dynamics of ischemia-related delayed death of neurons in CA1 area was investigated using light and electron microscopy. The conditions applied in this study induced the delayed pyramidal cell death associated with ultrastructural modifications in excitatory synapses on dendritic spines in the stratum radiatum of the CA1 hippocampal area.The morphological ischemia-evoked remodeling of synapses manifested in following: alteration in proportion of different types of synapses, a depletion of synaptic vesicles in presynaptic terminals and changes in their spatial arrangement; a rapid increase of the postsynaptic density (PSD) thickness and length, formation of concave synapses with perforated PSD during the first 24 h after ischemic episode, a pronounced increase of the glial coverage of both pre- and postsynaptic structures.
The relationship was found between ischemia induced structural changes in neurons and activity patterns of glial cells (astrocyte hypertrophy and hyperplasia of microglia).
Oxygen-glucose deprivation (OGD) of hippocampal cultured slices revealed that hippocampal CA1 pyramidal neurons are the most sensitive to oxygen-glucose deficit, while interneurons, astrocytes and microglia are more ischemia resistant and even get activated. Study of the certain signaling pathways selective blocking reveals possible modulating effect of interneurons on viability of pyramidal neurons in hippocampus. The level of expression of subunits HIF-1α, HIF-3α, SERCA2b and PMCA1-2 in neurons of CA1 and СА3 area of hippocampus changes in response to OGD and anoxic preconditioning. The number of damaged cells in both areas of the hippocampus reduces with a stabilization of HIF-1. Developing of the original image analyzing program allowed us to determine changes in spatial patterns formed by synaptic vesicles in the CA1 synapses. It was shown that distances between individual vesicles increased while the organelles became more distant from the presynaptic membrane. It was found that during early after an ischemic damage the ratio of synaptic terminal types changed, the frequency of perforated and multiple synapses being increased. These changes may indicate ischemia-related synaptic plasticity. While researching the protective approaches against brain ischemia there was obtained the data indicating the neuroprotective properties of aromatic aminoacids, FGL - NCAM peptide mimetic, bioflavonoids, particularly korvitin, that enabled to recommend these compounds for pharmacological applications. Study of exocrine pancreatic insufficiency (EPI) in pigs discovered the dependence of morphological and functional state of hippocampal neurons on pancreas exocrine functioning. Exogenous pancreatic-like enzymes are recovered in the gut and improve growth of exocrine pancreatic insufficient pigs.
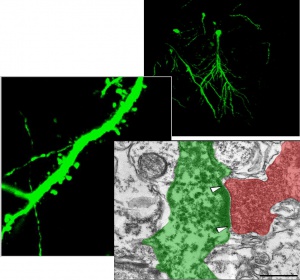
Regenerative capacity of different lines of stem cells is studied using in vivo and in vitro models of cerebral ischemia. In both models it was displayed that hippocampus-derived NPCs transplanted into the ischemic brain are able to survive for long terms after grafting, differentiate into functional mature neurons, become synaptically integrated into host circuitry. Syngeneic NPC transplantation promoted cognitive function recovery after ischemic injury in vivo.We have studied changes in proteasome proteolysis during transient occlusion of the middle cerebral artery in rats and made the comparison between changes in different types of proteasome activity and severity of ischemic injury and showed three types of decrease in proteolytic activity (trypsin-like, chymotrypsin-like, peptidyl-glutamyl peptide-hydrolyzing-like) in the brain tissues. These data suggest that ischemia may cause a severe alteration in proteasome stability and proteasome structure. Research resources of the Department are widely used for scientific practical training of students from Educational and Scientific Centre “Institute of Biology” of Taras Shevchenko National University of Kyiv, National University of Kyiv-Mohyla Academy and Kiev Branch of Moscow Institute of Physics and Technology.
Team
Stuff
- Skibo G.G. - Head of Department, MD, Prof. tel.+380 44 253-21-58, te.l./fax+380 44 256-24-42
- Kovalenko T.M. – PhD, Leading Researcher tel. + 380 44 256-24-43
- Nikonenko O.G. – PhD, Leading Researcher tel. + 380 44 256-25-70
- Tsupykov O.M – PhD, Leading Researcher tel. + 380 44 256-25-80
- Lushnikova I.V. – PhD, Senior Researcher tel. + 380 44 256 20 26
- Osadchenko I.O. – PhD, Senior Researcher tel. + 380 44 256-24-43
- Patseva М.А. – PhD, Researcher tel. + 380 44 256 24 58
- Nikandrova E.O. – Junior Researcher tel. + 380 44 256-20 26
- Savchuk O.I. – Junior Researcher tel. + 380 44 256-25-80
- Smozhanyk K.G. – Leading Engineer tel. + 380 44 256 24 45
- Mayorenko P.P. – Leading Engineer
- Melnychuk L.P. - Laboratory assistant
- Maleyeva G.
Research activities
Main areas of research
- viability and resistance of hippocampal neurons and glial cells under modeling of neuropathologies (cerebral ischemia, Alzheimer’s disease, stress); approaches for neuroprotection
- pathophysiological mechanisms of perinatal pathology of CNS and approaches for it therapeutic modulation;
- cell mechanisms of early neurodegeneration in rotenone-induced model of Parkinson’s disease;
- structural and functional peculiarities of the brain related to the gastrointestinal tract functioning;
- cell regenerative capacities for treatment of experimentally provoked brain neurodegeneration (ischemia, trauma).
Research objects
- Animals (rats, mice, gerbils, pigs)
- Cell culture (organ-like and dissociated culture)
Research methods
- modelling of neurodegeneration in vivo (cerebral ischemia, Parkinson’s disease, perinatal pathology of CNS, exocrine pancreatic insufficiency) and in vitro (cerebral ischemia, Alcheimer’s disease, stress);
- microscopy: light, confocal, electron;
- immunohystochemical methods;
- single-cell RT-PCR;
- immunoblotting;
- quantitative ultarstructural analysis;
- computerized image analysis;
- morphometry.
List of laboratories with which there is scientific cooperation
- Department of Cell and Organism Biology, Lund University, Sweden;
- Laboratory of Neural Stem Cell Biology&Therapy, Lund Stem Cell Center, Sweden;
- Institute of brain dynamics National Institute for Health and Medical Research (INSERM), Marseille, France;
Scientific advances. Selected publications for years 2013-2017
- Kanemitsu M, Tsupykov O, Potter G, Boitard M, Salmon P, Zgraggen E, Gascon E, Skibo G, Dayer AG, Kiss JZ. EMMPRIN overexpression in SVZ neural progenitor cells increases their migration towards ischemic cortex Exp Neurol. 2017 Nov;297:14-24. (https://www.sciencedirect.com/science/article/pii/S001448861730184X?via%3Dihub, IF 4,7)
- Zoltowska KM, Maesako M, Lushnikova I, Takeda S, Keller LJ, Skibo G, Hyman BT, Berezovska O. Dynamic presenilin 1 and synaptotagmin 1 interaction modulates exocytosis and amyloid β production. Mol Neurodegener. 2017 Feb 13;12(1):15. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5307796/, IF 6,78)
- Tornero D, Tsupykov O, Granmo M, Rodriguez C, Grønning-Hansen M, Thelin J, Smozhanik E, Laterza C, Wattananit S, Ge R, Tatarishvili J, Grealish S, Brüstle O, Skibo G, Parmar M, Schouenborg J, Lindvall O, Kokaia Z. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017 Mar 1;140(3):692-706. (https://academic.oup.com/brain/article-abstract/140/3/692/2936871?redirectedFrom=fulltext, IF 10,29)
- Gerth F, Pechstein A, Kochlamazashvili G, Jäpel M, Lehmann M, Puchkov D, Onofri F, Benfenati F, Nikonenko AG, Maritzen T, Freund Ch, Haucke V. Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):12057-12062. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5692602/, IF 9,7)
- Kopach O, Maistrenko A, Lushnikova I, Belan P, Skibo G, Voitenko N. HIF-1α-mediated upregulation of SERCA2b: The endogenous mechanism for alleviating the ischemia-induced intracellular Ca(2+) store dysfunction in CA1 and CA3 hippocampal neurons. Cell Calcium. 2016 May;59(5):251-61. (https://www.sciencedirect.com/science/article/pii/S0143416016300173?via%3Dihub, IF 3,43)
- Tsupykov O, Kanemitsu M, Smozhanik E, Skibo G, Dayer AG, Kiss JZ. Relationship of Grafted FGF-2-Overexpressing Neural Stem/Progenitor Cells With the Vasculature in the Cerebral Cortex. Cell Transplant. 2016;25(7):1359-69. (http://journals.sagepub.com/doi/abs/10.3727/096368916X690421?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed&, IF 3,43)
- Nikonenko I, Nikonenko A, Mendez P, Michurina TV, Enikolopov G, Muller D. Nitric oxide mediates local activity-dependent excitatory synapse development. Proc Natl Acad Sci U S A. 2013, 110(44):E4142-4151. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3816470/, 9,74)