Department of Cytology
Інститут фізіології ім. О. О. Богомольця
(Difference between revisions)
| (22 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Department of Cytology has been established in 1996 based on the Laboratory of Neurocytology. Its organizer and the only chair until nowadays is [G.G.Skibo|Prof. Galyna Skibo], the corresponding member of NAS of Ukraine, laureate of State Prize of Ukraine in sciences and engineering, Bogomoletz and Kostiuk Prizes of NAS of Ukraine. | |
| − | + | ||
| − | + | == Our research == | |
| − | </div> | + | |
| + | The research activity of the Department of Cytology is focused on the study of cellular and molecular mechanisms of neurodegeneration in different parts of the nervous system and the search for neuroprotective approaches. The current research topics of the Department are: | ||
| + | * genetically determined molecular mechanisms of intercellular and intracellular signaling in norm and pathology in vitro (brain ischemia, Alzheimer’s disease) | ||
| + | * molecular and cellular mechanisms of endogenous neuroprotection in the models of neurodegenerative diseases in vivo and in vitro (brain ischemia) | ||
| + | * regenerative properties of different origin stem cells and peculiarities of their application at experimentally induced neurodegenerative pathologies in vivo (brain ischemia, perinatal CNS pathology, neuroinflammation) | ||
| + | * development of biosafety criteria for stem cell cultivation | ||
| + | * cell mechanisms driving brain tissue degeneration in the rotenone model of Parkinson's disease | ||
| + | * dependence of structural and functional features of the brain on the function of the gastrointestinal tract (metabolic syndrome, exocrine pancreatic insufficiency) in vivo and in vitro | ||
| + | * effect of substances with antioxidant properties on the nervous tissue recovery of experimental animals during the brain ischemia-reperfusion | ||
| + | <br clear=all> | ||
| + | |||
| + | ---- | ||
| + | |||
| + | <div><ul> | ||
| + | <li style="display: inline-block;"> [[File:Dept_cytol_01.jpeg|thumb|none|300px|Synapses with continuous (A) and perforated (B) postsynaptic densities (red lines) between host dendritic spines (ds, brown) and grafted GFP+/DAB+ presynaptic axon terminals (at, green) in the contralateral somatosensory cortex of stroke-injured rats. (Scale bar – 0.2 μm) [https://doi.org/10.1073/pnas.2000690117 Source] ]] </li> | ||
| + | <li style="display: inline-block;"> [[File:Dept_cytol_02.gif |thumb|none|300px|The GIF animation created using serial electron micrographs of the hippocampal CA1 area, illustrating the direct interaction of the pyramidal neuron and astrocyte by exocytosis/endocytosis (to see that animation, open full image) [https://doi.org/10.1007/s12264-021-00725-5 Source] ]] </li> | ||
| + | <li style="display: inline-block;"> [[File:Dept_cytol_03.jpeg|thumb|none|300px|FGF-2-Overexpressing Neural Stem/Progenitor Cells (FGF-2-NPCs) made contact with blood vessels at sites with astrocyte coverage, but no pericytes [https://doi.org/10.3727%2F096368916X690421 Source] ]] </li> | ||
| + | <li style="display: inline-block;"> [[File:Dept_cytol_04.jpeg|thumb|none|300px|Synapses in СА1 stratum radiatum in vivo | ||
| + | Red arrow-simple, yellow-multiple, green-perforated [https://doi.org/10.1002/hipo.20211 Source] ]] </li> | ||
| + | <li style="display: inline-block;"> [[File:Dept_cytol_05.jpeg|thumb|none|300px|3D reconstructions of spine synapses contacted by glia under ischemic conditions [https://doi.org/10.1002/hipo.20551 Source] ]] </li> | ||
| + | </ul></div> | ||
| + | |||
| + | ==Research objects== | ||
| + | * Animals: rats, mice, gerbils, pigs; | ||
| + | * Cell cultures: organotypic cultures of the hippocampus, dissociated cultures of CNS neurons, enteric neuron cultures. | ||
| + | |||
| + | ==Research methods== | ||
| + | * modelling of neurodegenerative states in vivo (cerebral ischemia, Parkinson’s disease, neuroinflammation, perinatal pathology of CNS, metabolic syndrome, exocrine pancreatic insufficiency) and in vitro (cerebral ischemia, Alzheimer’s disease, stress); | ||
| + | * light, confocal, and transmission electron microscopy; | ||
| + | * immunohistochemistry; | ||
| + | * behavioral tests (open field, balance beam walking, Morris water maze, T- maze) | ||
| + | * immunoblotting; | ||
| + | * morphometry: computerized image analysis, quantitative ultrastructural analysis. | ||
| + | |||
| + | ==Collaboration== | ||
| + | * Lund Stem Cell Center, Lund University, Lund, Sweden; | ||
| + | * Food for Health Science Center, Lund University, Lund, Sweden; | ||
| + | * Chebotarev State Institute of Gerontology NAMS of Ukraine, Kyiv, Ukraine; | ||
| + | * Oles Honchar Dnipro National University, Dnipro, Ukraine. | ||
| + | |||
| + | == Team == | ||
| + | |||
| + | # Galyna Skibo – Prof., DSc, corresponding member of NAS of Ukraine, head of Department, skibo@biph.kiev.ua | ||
| + | # Oleksandr Nikonenko – DSc, leading researcher, agn@biph.kiev.ua | ||
| + | # Oleh Tsupykov – DSc, leading researcher, tsupykov@gmail.com | ||
| + | # Iryna Lushnikova – DSc, leading researcher, li@biph.kiev.ua | ||
| + | # Tetiana Kovalenko – Candidate of sciences (PhD), senior researcher, tnk@biph.kiev.ua | ||
| + | # Iryna Osadchenko – Candidate of sciences (PhD), senior researcher, osad@biph.kiev.ua | ||
| + | # Olena Mankivska – Candidate of sciences (PhD), senior researcher emankovskaya@biph.kiev.ua | ||
| + | # Kateryna Yatsenko – DSc, senior researcher, kateryna.yatsenko@gmail.com | ||
| + | # Maryna Patseva – Candidate of sciences (PhD), researcher, pma@biph.kiev.ua | ||
| + | # Olena Savchuk – Candidate of sciences (PhD), junior researcher, floweringbowl@ukr.net | ||
| + | # Kateryna Smozhanyk – technician. | ||
| + | # Petro Maiorenko – technician. | ||
| + | # Liudmyla Melnychuk - laboratory assistant. | ||
| + | |||
| + | ===PhD students=== | ||
| + | |||
| + | # Nataliia Chaika - nataliia_chaika@ukr.net | ||
| + | # Olha Kostiuchenko - kostiuchenko.olha@biph.kiev.ua | ||
| + | # Nadiia Kravchenko - kravchenko.nadiia@biph.kiev.ua | ||
| + | # Dmytro Shepilov - shepilov@biph.kiev.ua | ||
| + | |||
| + | == Recent publications == | ||
| + | |||
| + | * Shepilov D, Kovalenko T, Osadchenko I, Smozhanyk K, Marungruang N, Ushakova G, Muraviova D, Hållenius F, Prykhodko O, Skibo G. [https://doi.org/10.3389/fnut.2022.565051 Varying Dietary Component Ratios and Lingonberry Supplementation May Affect the Hippocampal Structure of ApoE-/- Mice.] Front Nutr. 2022 Feb 16;9:565051. doi.org/10.3389/fnut.2022.565051 | ||
| + | |||
| + | * Mankivska, O.P., Chaika, N.V. & Skibo, G.G. [https://doi.org/10.1007/s11062-022-09918-8 Effects of Citicoline on Structural/Functional Consequences of Focal Ischemia of the Rat Brain]. Neurophysiology. 2022; 53, 78–87. doi.org/10.1007/s11062-022-09918-8 | ||
| + | |||
| + | * Govbakh I, Kyryk V, Ustymenko A, Rubtsov V, Tsupykov O, Bulgakova NV, Zavodovskiy DO, Sokolowska I, Maznychenko A. [https://doi.org/10.3390/ijms222112026 Stem Cell Therapy Enhances Motor Activity of Triceps Surae Muscle in Mice with Hereditary Peripheral Neuropathy]. Int J Mol Sci. 2021 Nov 6;22(21):12026. doi.org/10.3390/ijms222112026 | ||
| + | |||
| + | * Balatskyi VV, Vaskivskyi VO, Myronova A, Avramets D, Nahia KA, Macewicz LL, Ruban TP, Kucherenko DY, Soldatkin OO, Lushnikova IV, Skibo GG, Winata CL, Dobrzyn P, Piven OO. [https://doi.org/10.1016/j.mito.2021.07.005 Сardiac-specific β-catenin deletion dysregulates energetic metabolism and mitochondrial function in perinatal cardiomyocytes]. Mitochondrion. 2021 Sep;60:59-69. doi.org/10.1016/j.mito.2021.07.005 | ||
| + | |||
| + | * Götz TWB, Puchkov D, Lysiuk V, Lützkendorf J, Nikonenko AG, Quentin C, Lehmann M, Sigrist SJ, Petzoldt AG. [https://doi.org/10.1083/jcb.202006040 Rab2 regulates presynaptic precursor vesicle biogenesis at the trans-Golgi]. J Cell Biol. 2021 May 3;220(5):e202006040. doi.org/10.1083/jcb.202006040 | ||
| + | |||
| + | * Lushnikova I, Nikandrova Y, Skibo G. [https://doi.org/10.1007/s12264-021-00725-5 Mitochondrial Events Determine the Status of Hippocampal Cells in the Post-Ischemic Period]. Neurosci Bull. 2021 Aug;37(8):1246-1250. doi.org/10.1007/s12264-021-00725-5 | ||
| + | |||
| + | * Bozhok YM, Golovko O, Nikonenko AG. [https://doi.org/10.1016/j.cmpb.2020.105562 nPAsym: an open-source plugin for ImageJ to quantify nuclear shape asymmetry]. Comput Methods Programs Biomed. 2020;196:105562. doi.org/10.1016/j.cmpb.2020.105562 | ||
| + | |||
| + | * Marungruang N, Kovalenko T, Osadchenko I, Voss U, Huang F, Burleigh S, Ushakova G, Skibo G, Nyman M, Prykhodko O, Hållenius FF. [https://doi.org/10.1080/1028415x.2018.1536423 Lingonberries and their two separated fractions differently alter the gut microbiota, improve metabolic functions, reduce gut inflammatory properties, and improve brain function in ApoE-/- mice fed high-fat diet]. Nutr Neurosci. 2020;23(8):600-612. doi.org/10.1080/1028415X.2018.1536423 | ||
| + | |||
| + | * Yatsenko K, Lushnikova I, Ustymenko A, Patseva M, Govbakh I, Kyryk V, Tsupykov O. [https://doi.org/10.3390/jpm10030066 Adipose-Derived Stem Cells Reduce Lipopolysaccharide-Induced Myelin Degradation and Neuroinflammatory Responses of Glial Cells in Mice]. J Pers Med. 2020;10(3):66 doi.org/10.3390/jpm10030066 | ||
| + | |||
| + | * Maznychenko AV, Bulgakova NV, Sokolowska IV, Butowska K, Borowik A, Mankivska OP, Piosik J, Tomiak T, Gonchar OO, Maisky VO, Kostyukov AI. [https://doi.org/10.1038/s41598-020-67034-1 Fatigue-induced Fos immunoreactivity within the lumbar cord and amygdala decreases after С60 fullerene pretreatment]. Sci Rep. 2020;10(1):9826. (https://www.nature.com/articles/s41598-020-67034-1 | ||
| + | |||
| + | * Grønning Hansen M, Laterza C, Palma-Tortosa S, Kvist G, Monni E, Tsupykov O, Tornero D, Uoshima N, Soriano J, Bengzon J, Martino G, Skibo G, Lindvall O, Kokaia Z. [https://doi.org/10.1002/sctm.20-0134 Grafted human pluripotent stem cell-derived cortical neurons integrate into adult human cortical neural circuitry]. Stem Cells Transl Med. 2020;9(11):1365-1377. doi.org/10.1002/sctm.20-0134 | ||
| + | |||
| + | * Palma-Tortosa S, Tornero D, Grønning Hansen M, Monni E, Hajy M, Kartsivadze S, Aktay S, Tsupykov O, Parmar M, Deisseroth K, Skibo G, Lindvall O, Kokaia Z. [https://doi.org/10.1073/pnas.2000690117 Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior]. Proc Natl Acad Sci U S A. 2020;117(16):9094-9100. doi.org/10.1073/pnas.2000690117 | ||
| + | |||
| + | * Kanemitsu M, Tsupykov O, Potter G, Boitard M, Salmon P, Zgraggen E, Gascon E, Skibo G, Dayer AG, Kiss JZ. [https://doi.org/10.1016/j.expneurol.2017.07.009 EMMPRIN overexpression in SVZ neural progenitor cells increases their migration towards ischemic cortex]. Exp Neurol. 2017 Nov;297:14-24. doi.org/10.1016/j.expneurol.2017.07.009 | ||
| + | |||
| + | * Zoltowska KM, Maesako M, Lushnikova I, Takeda S, Keller LJ, Skibo G, Hyman BT, Berezovska O. [https://doi.org/10.1186/s13024-017-0159-y Dynamic presenilin 1 and synaptotagmin 1 interaction modulates exocytosis and amyloid β production]. Mol Neurodegener. 2017 Feb 13;12(1):15 dx.doi.org/10.1186%2Fs13024-017-0159-y | ||
| + | |||
| + | * Tornero D, Tsupykov O, Granmo M, Rodriguez C, Grønning-Hansen M, Thelin J, Smozhanik E, Laterza C, Wattananit S, Ge R, Tatarishvili J, Grealish S, Brüstle O, Skibo G, Parmar M, Schouenborg J, Lindvall O, Kokaia Z. [https://doi.org/10.1093/brain/aww347 Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli]. Brain. 2017 Mar 1;140(3):692-706. /doi.org/10.1093/brain/aww347 | ||
| + | |||
| + | * Gerth F, Pechstein A, Kochlamazashvili G, Jäpel M, Lehmann M, Puchkov D, Onofri F, Benfenati F, Nikonenko AG, Maritzen T, Freund Ch, Haucke V. [https://doi.org/10.1073/pnas.1715341114 Intersectin associates with synapsin and regulates its nanoscale localization and function]. Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):12057-12062. dx.doi.org/10.1073%2Fpnas.1715341114 | ||
| + | |||
| + | * Kopach O, Maistrenko A, Lushnikova I, Belan P, Skibo G, Voitenko N. [https://doi.org/10.1016/j.ceca.2016.02.014 HIF-1α-mediated upregulation of SERCA2b: The endogenous mechanism for alleviating the ischemia-induced intracellular Ca(2+) store dysfunction in CA1 and CA3 hippocampal neurons]. Cell Calcium. 2016. May;59(5):251-61. doi.org/10.1016/j.ceca.2016.02.014 | ||
| + | |||
| + | * Tsupykov O, Kanemitsu M, Smozhanik E, Skibo G, Dayer AG, Kiss JZ. [https://doi.org/10.3727/096368916x690421 Relationship of Grafted FGF-2-Overexpressing Neural Stem/Progenitor Cells With the Vasculature in the Cerebral Cortex]. Cell Transplant. 2016;25(7):1359-69. doi.org/10.3727%2F096368916X690421 | ||
Latest revision as of 08:24, 27 June 2022
Department of Cytology has been established in 1996 based on the Laboratory of Neurocytology. Its organizer and the only chair until nowadays is [G.G.Skibo|Prof. Galyna Skibo], the corresponding member of NAS of Ukraine, laureate of State Prize of Ukraine in sciences and engineering, Bogomoletz and Kostiuk Prizes of NAS of Ukraine.
Contents |
Our research
The research activity of the Department of Cytology is focused on the study of cellular and molecular mechanisms of neurodegeneration in different parts of the nervous system and the search for neuroprotective approaches. The current research topics of the Department are:
- genetically determined molecular mechanisms of intercellular and intracellular signaling in norm and pathology in vitro (brain ischemia, Alzheimer’s disease)
- molecular and cellular mechanisms of endogenous neuroprotection in the models of neurodegenerative diseases in vivo and in vitro (brain ischemia)
- regenerative properties of different origin stem cells and peculiarities of their application at experimentally induced neurodegenerative pathologies in vivo (brain ischemia, perinatal CNS pathology, neuroinflammation)
- development of biosafety criteria for stem cell cultivation
- cell mechanisms driving brain tissue degeneration in the rotenone model of Parkinson's disease
- dependence of structural and functional features of the brain on the function of the gastrointestinal tract (metabolic syndrome, exocrine pancreatic insufficiency) in vivo and in vitro
- effect of substances with antioxidant properties on the nervous tissue recovery of experimental animals during the brain ischemia-reperfusion
-
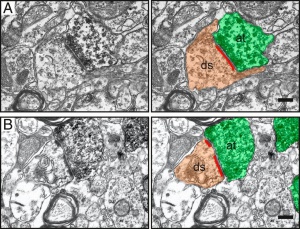 Synapses with continuous (A) and perforated (B) postsynaptic densities (red lines) between host dendritic spines (ds, brown) and grafted GFP+/DAB+ presynaptic axon terminals (at, green) in the contralateral somatosensory cortex of stroke-injured rats. (Scale bar – 0.2 μm) Source
Synapses with continuous (A) and perforated (B) postsynaptic densities (red lines) between host dendritic spines (ds, brown) and grafted GFP+/DAB+ presynaptic axon terminals (at, green) in the contralateral somatosensory cortex of stroke-injured rats. (Scale bar – 0.2 μm) Source -
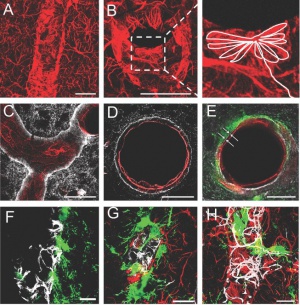
 The GIF animation created using serial electron micrographs of the hippocampal CA1 area, illustrating the direct interaction of the pyramidal neuron and astrocyte by exocytosis/endocytosis (to see that animation, open full image) Source
The GIF animation created using serial electron micrographs of the hippocampal CA1 area, illustrating the direct interaction of the pyramidal neuron and astrocyte by exocytosis/endocytosis (to see that animation, open full image) Source -
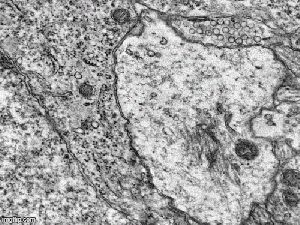
 FGF-2-Overexpressing Neural Stem/Progenitor Cells (FGF-2-NPCs) made contact with blood vessels at sites with astrocyte coverage, but no pericytes Source
FGF-2-Overexpressing Neural Stem/Progenitor Cells (FGF-2-NPCs) made contact with blood vessels at sites with astrocyte coverage, but no pericytes Source -
 Synapses in СА1 stratum radiatum in vivo Red arrow-simple, yellow-multiple, green-perforated Source
Synapses in СА1 stratum radiatum in vivo Red arrow-simple, yellow-multiple, green-perforated Source -
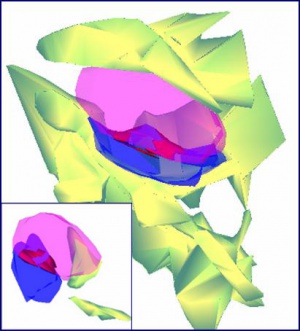 3D reconstructions of spine synapses contacted by glia under ischemic conditions Source
3D reconstructions of spine synapses contacted by glia under ischemic conditions Source
Research objects
- Animals: rats, mice, gerbils, pigs;
- Cell cultures: organotypic cultures of the hippocampus, dissociated cultures of CNS neurons, enteric neuron cultures.
Research methods
- modelling of neurodegenerative states in vivo (cerebral ischemia, Parkinson’s disease, neuroinflammation, perinatal pathology of CNS, metabolic syndrome, exocrine pancreatic insufficiency) and in vitro (cerebral ischemia, Alzheimer’s disease, stress);
- light, confocal, and transmission electron microscopy;
- immunohistochemistry;
- behavioral tests (open field, balance beam walking, Morris water maze, T- maze)
- immunoblotting;
- morphometry: computerized image analysis, quantitative ultrastructural analysis.
Collaboration
- Lund Stem Cell Center, Lund University, Lund, Sweden;
- Food for Health Science Center, Lund University, Lund, Sweden;
- Chebotarev State Institute of Gerontology NAMS of Ukraine, Kyiv, Ukraine;
- Oles Honchar Dnipro National University, Dnipro, Ukraine.
Team
- Galyna Skibo – Prof., DSc, corresponding member of NAS of Ukraine, head of Department, skibo@biph.kiev.ua
- Oleksandr Nikonenko – DSc, leading researcher, agn@biph.kiev.ua
- Oleh Tsupykov – DSc, leading researcher, tsupykov@gmail.com
- Iryna Lushnikova – DSc, leading researcher, li@biph.kiev.ua
- Tetiana Kovalenko – Candidate of sciences (PhD), senior researcher, tnk@biph.kiev.ua
- Iryna Osadchenko – Candidate of sciences (PhD), senior researcher, osad@biph.kiev.ua
- Olena Mankivska – Candidate of sciences (PhD), senior researcher emankovskaya@biph.kiev.ua
- Kateryna Yatsenko – DSc, senior researcher, kateryna.yatsenko@gmail.com
- Maryna Patseva – Candidate of sciences (PhD), researcher, pma@biph.kiev.ua
- Olena Savchuk – Candidate of sciences (PhD), junior researcher, floweringbowl@ukr.net
- Kateryna Smozhanyk – technician.
- Petro Maiorenko – technician.
- Liudmyla Melnychuk - laboratory assistant.
PhD students
- Nataliia Chaika - nataliia_chaika@ukr.net
- Olha Kostiuchenko - kostiuchenko.olha@biph.kiev.ua
- Nadiia Kravchenko - kravchenko.nadiia@biph.kiev.ua
- Dmytro Shepilov - shepilov@biph.kiev.ua
Recent publications
- Shepilov D, Kovalenko T, Osadchenko I, Smozhanyk K, Marungruang N, Ushakova G, Muraviova D, Hållenius F, Prykhodko O, Skibo G. Varying Dietary Component Ratios and Lingonberry Supplementation May Affect the Hippocampal Structure of ApoE-/- Mice. Front Nutr. 2022 Feb 16;9:565051. doi.org/10.3389/fnut.2022.565051
- Mankivska, O.P., Chaika, N.V. & Skibo, G.G. Effects of Citicoline on Structural/Functional Consequences of Focal Ischemia of the Rat Brain. Neurophysiology. 2022; 53, 78–87. doi.org/10.1007/s11062-022-09918-8
- Govbakh I, Kyryk V, Ustymenko A, Rubtsov V, Tsupykov O, Bulgakova NV, Zavodovskiy DO, Sokolowska I, Maznychenko A. Stem Cell Therapy Enhances Motor Activity of Triceps Surae Muscle in Mice with Hereditary Peripheral Neuropathy. Int J Mol Sci. 2021 Nov 6;22(21):12026. doi.org/10.3390/ijms222112026
- Balatskyi VV, Vaskivskyi VO, Myronova A, Avramets D, Nahia KA, Macewicz LL, Ruban TP, Kucherenko DY, Soldatkin OO, Lushnikova IV, Skibo GG, Winata CL, Dobrzyn P, Piven OO. Сardiac-specific β-catenin deletion dysregulates energetic metabolism and mitochondrial function in perinatal cardiomyocytes. Mitochondrion. 2021 Sep;60:59-69. doi.org/10.1016/j.mito.2021.07.005
- Götz TWB, Puchkov D, Lysiuk V, Lützkendorf J, Nikonenko AG, Quentin C, Lehmann M, Sigrist SJ, Petzoldt AG. Rab2 regulates presynaptic precursor vesicle biogenesis at the trans-Golgi. J Cell Biol. 2021 May 3;220(5):e202006040. doi.org/10.1083/jcb.202006040
- Lushnikova I, Nikandrova Y, Skibo G. Mitochondrial Events Determine the Status of Hippocampal Cells in the Post-Ischemic Period. Neurosci Bull. 2021 Aug;37(8):1246-1250. doi.org/10.1007/s12264-021-00725-5
- Bozhok YM, Golovko O, Nikonenko AG. nPAsym: an open-source plugin for ImageJ to quantify nuclear shape asymmetry. Comput Methods Programs Biomed. 2020;196:105562. doi.org/10.1016/j.cmpb.2020.105562
- Marungruang N, Kovalenko T, Osadchenko I, Voss U, Huang F, Burleigh S, Ushakova G, Skibo G, Nyman M, Prykhodko O, Hållenius FF. Lingonberries and their two separated fractions differently alter the gut microbiota, improve metabolic functions, reduce gut inflammatory properties, and improve brain function in ApoE-/- mice fed high-fat diet. Nutr Neurosci. 2020;23(8):600-612. doi.org/10.1080/1028415X.2018.1536423
- Yatsenko K, Lushnikova I, Ustymenko A, Patseva M, Govbakh I, Kyryk V, Tsupykov O. Adipose-Derived Stem Cells Reduce Lipopolysaccharide-Induced Myelin Degradation and Neuroinflammatory Responses of Glial Cells in Mice. J Pers Med. 2020;10(3):66 doi.org/10.3390/jpm10030066
- Maznychenko AV, Bulgakova NV, Sokolowska IV, Butowska K, Borowik A, Mankivska OP, Piosik J, Tomiak T, Gonchar OO, Maisky VO, Kostyukov AI. Fatigue-induced Fos immunoreactivity within the lumbar cord and amygdala decreases after С60 fullerene pretreatment. Sci Rep. 2020;10(1):9826. (https://www.nature.com/articles/s41598-020-67034-1
- Grønning Hansen M, Laterza C, Palma-Tortosa S, Kvist G, Monni E, Tsupykov O, Tornero D, Uoshima N, Soriano J, Bengzon J, Martino G, Skibo G, Lindvall O, Kokaia Z. Grafted human pluripotent stem cell-derived cortical neurons integrate into adult human cortical neural circuitry. Stem Cells Transl Med. 2020;9(11):1365-1377. doi.org/10.1002/sctm.20-0134
- Palma-Tortosa S, Tornero D, Grønning Hansen M, Monni E, Hajy M, Kartsivadze S, Aktay S, Tsupykov O, Parmar M, Deisseroth K, Skibo G, Lindvall O, Kokaia Z. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci U S A. 2020;117(16):9094-9100. doi.org/10.1073/pnas.2000690117
- Kanemitsu M, Tsupykov O, Potter G, Boitard M, Salmon P, Zgraggen E, Gascon E, Skibo G, Dayer AG, Kiss JZ. EMMPRIN overexpression in SVZ neural progenitor cells increases their migration towards ischemic cortex. Exp Neurol. 2017 Nov;297:14-24. doi.org/10.1016/j.expneurol.2017.07.009
- Zoltowska KM, Maesako M, Lushnikova I, Takeda S, Keller LJ, Skibo G, Hyman BT, Berezovska O. Dynamic presenilin 1 and synaptotagmin 1 interaction modulates exocytosis and amyloid β production. Mol Neurodegener. 2017 Feb 13;12(1):15 dx.doi.org/10.1186%2Fs13024-017-0159-y
- Tornero D, Tsupykov O, Granmo M, Rodriguez C, Grønning-Hansen M, Thelin J, Smozhanik E, Laterza C, Wattananit S, Ge R, Tatarishvili J, Grealish S, Brüstle O, Skibo G, Parmar M, Schouenborg J, Lindvall O, Kokaia Z. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017 Mar 1;140(3):692-706. /doi.org/10.1093/brain/aww347
- Gerth F, Pechstein A, Kochlamazashvili G, Jäpel M, Lehmann M, Puchkov D, Onofri F, Benfenati F, Nikonenko AG, Maritzen T, Freund Ch, Haucke V. Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):12057-12062. dx.doi.org/10.1073%2Fpnas.1715341114
- Kopach O, Maistrenko A, Lushnikova I, Belan P, Skibo G, Voitenko N. HIF-1α-mediated upregulation of SERCA2b: The endogenous mechanism for alleviating the ischemia-induced intracellular Ca(2+) store dysfunction in CA1 and CA3 hippocampal neurons. Cell Calcium. 2016. May;59(5):251-61. doi.org/10.1016/j.ceca.2016.02.014
- Tsupykov O, Kanemitsu M, Smozhanik E, Skibo G, Dayer AG, Kiss JZ. Relationship of Grafted FGF-2-Overexpressing Neural Stem/Progenitor Cells With the Vasculature in the Cerebral Cortex. Cell Transplant. 2016;25(7):1359-69. doi.org/10.3727%2F096368916X690421
