Department of Molecular biophysics
Amor (Обговорення • внесок) м (→Graduate Biophysics and Physiology) |
Amor (Обговорення • внесок) (→Facilities) |
||
| Рядок 178: | Рядок 178: | ||
=='''Facilities'''== | =='''Facilities'''== | ||
| − | Laboratory of Molecular Biophysics is equipped with three imaging rigs: | + | '''Laboratory of Molecular Biophysics''' is equipped with three imaging rigs: |
| − | Two high-speed epifluorescence imaging stations mounted on inverted fluorescent microscopes (Olympus IX-70/71, Olympus, Japan) and monochromator Polychrome V (Till Photonics, Germany), CCD camera SVGA (Imago, Till Photonics, Germany), a computer graphic station and imaging software LA and Till vision (Till Photonics, Germany); one of the setup is combined with a DualView system (Optical Insights, USA) necessary to perform fast FRET recordings. The stations are equipped with electrophysiological equipment, including two double patch-clamp amplifiers EPC-10/2 (HEKA Elektronik, Germany), patch-pipette puller P-97 (Sutter, USA), two high-precision micromanipulator MP-285 (Sutter, USA) and two hydraulic micromanipulator (Narishige, Japan) and air tables (TMC and Newport, USA). | + | |
| − | Confocal microscope imaging station Fluoview 1000 (Olympus, Japan) having UV, 405, 488, 515, 543 and 633 nm laser lines and mounted on an upright BX61WI microscope (Olympus) (this is shared equipment). It is mounted on an air table and bundled with patch-clamp amplifier Dagan 3900A (Dagan Corporation, USA), AD/DA board (National Instruments, USA), motorized stage (MMTS, Scientifica, UK) and set of micromanipulators (PatchStar, Scientifica, UK), IsoFlex extracellular stimulator (A.M.P.I., Israel) and temperature-controlled perfusion system (Warrner Instruments, USA). | + | *Two high-speed epifluorescence imaging stations mounted on inverted fluorescent microscopes (Olympus IX-70/71, Olympus, Japan) and monochromator Polychrome V (Till Photonics, Germany), CCD camera SVGA (Imago, Till Photonics, Germany), a computer graphic station and imaging software LA and Till vision (Till Photonics, Germany); one of the setup is combined with a DualView system (Optical Insights, USA) necessary to perform fast FRET recordings. *The stations are equipped with electrophysiological equipment, including two double patch-clamp amplifiers EPC-10/2 (HEKA Elektronik, Germany), patch-pipette puller P-97 (Sutter, USA), two high-precision micromanipulator MP-285 (Sutter, USA) and two hydraulic micromanipulator (Narishige, Japan) and air tables (TMC and Newport, USA). |
| − | The Laboratory is also equipped with a fume hood and other laboratory supplies, including a dissection microscope, pH-meter, osmometer, balances, etc. The laboratory has a real-time qPCR machine, DNA Engine thermal cycler and Nanodrop instrument within 200 feet of lab for common use. | + | *Confocal microscope imaging station Fluoview 1000 (Olympus, Japan) having UV, 405, 488, 515, 543 and 633 nm laser lines and mounted on an upright BX61WI microscope (Olympus) (this is shared equipment). It is mounted on an air table and bundled with patch-clamp amplifier Dagan 3900A (Dagan Corporation, USA), AD/DA board (National Instruments, USA), motorized stage (MMTS, Scientifica, UK) and set of micromanipulators (PatchStar, Scientifica, UK), IsoFlex extracellular stimulator (A.M.P.I., Israel) and temperature-controlled perfusion system (Warrner Instruments, USA). |
| − | Tissue Culture Room has two biosafety hoods in space adjacent to his laboratory, as well as 3 cell culture incubators, refrigerators for culture reagents, inverted microscopes and other standard cell culture equipment. The Laboratory has access to shared liquid nitrogen storage for cell lines. | + | *The Laboratory is also equipped with a fume hood and other laboratory supplies, including a dissection microscope, pH-meter, osmometer, balances, etc. *The laboratory has a real-time qPCR machine, DNA Engine thermal cycler and Nanodrop instrument within 200 feet of lab for common use. |
| + | *Tissue Culture Room has two biosafety hoods in space adjacent to his laboratory, as well as 3 cell culture incubators, refrigerators for culture reagents, inverted microscopes and other standard cell culture equipment. The Laboratory has access to shared liquid nitrogen storage for cell lines. | ||
Версія за 20:12, 25 листопада 2015
Зміст |
About
Laboratory of Molecular Biophysics has been established in April 2012 based on the research group headed by Dr. P. Belan at the Department of General Physiology of the Nervous System. Regulation of intracellular cytoplasmic Ca2+ concentration and Ca2+ signalling in different types of excitable cells as well as modulation of synaptic transmission in norm and pathology are the main subject of our research during recent years. Acutely isolated and primary cultured neurons as well as slices and ex vivo intact spinal cord preparation have been used in the experiments. Mainly, optical (digital imaging and confocal microscopy), conventional electrophysiological (microelectrodes, iontophoresis, patch clamp), genetic (transfection, infection, site-specific mutagenesis) and mathematical (simulation, statistics) methods and approaches have been employed in our studies.
Research
Signalling of neuronal Ca2+ sensor proteins
Decoding glutamate receptor activation by the Ca2+ sensor proteins
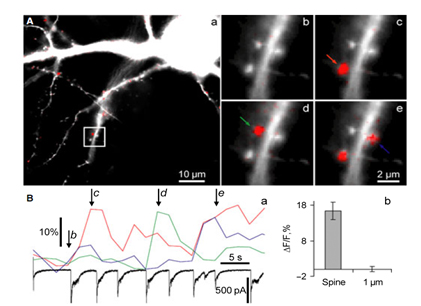
We study biophysical and physiological mechanisms of NCS (Neuronal Calcium Sensor) protein signaling in hippocampal neurons. Decoding of complex spatio-temporal patterns of [Ca2+]i changes by a Ca2+ sensor proteins, hippocalcin and neurocalcin δ, and involvement of Ca2+-dependent protein translocation in neuronal signal transduction is in a center of these studies. The most important achievement in this line of research is clear understanding that hippocalcin and neurocalcin δ may differentially decode various spatiotemporal patterns of glutamate receptor activation into site- and time-specific translocation to their membranous targets. Hippocalcin also possesses an ability to produce local signalling at the single synaptic level providing a molecular mechanism for homosynaptic plasticity (Dovgan et al., 2010). Examples of spontaneous hippocalcin-YFP translocation to a set of spines correlated with bursts of postsynaptic currents are shown in the Figure 1.
Analysis of the mechanisms underlying GABAergic synaptic transmission
Short-term plasticity of GABAergic synaptic transmission in the hippocampus
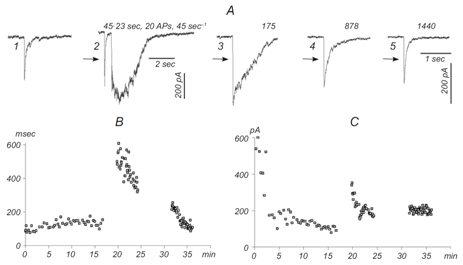
This part of our research is devoted to studies of different synaptic mechanisms contributing to GABAergic synaptic transmission in the hippocampus. Mechanisms of short-term depression and non-phasic vesicular release are in focus of our current research (Stepanyuk et al., 2014b)(Borisyuk et al., 2014). We have recently found a new type of short-term plasticity in cultured hippocampal neurons manifested as activity-dependent potentiation of an asynchronous component of GABAergic synaptic currents, аIPSCs (Fig. 2) (Borisyuk et al., 2014).
Simultaneously with potentiation of аIPSC, we observed plateau-like inward currents in the presynaptic neuron. The charge transferred by this current correlated significantly with the decay time of evoked IPSC in the postsynaptic neuron (mean correlation coefficient 0.83±0.10) suggesting that the inward current mediates аIPSC potentiation. We hypothesize that the observed plasticity may endogenously regulate the efficacy of GABAergic synaptic transmission in the hippocampus. In the ongoing research we are studying pre- and postsynaptic mechanisms involved in this new type of inhibitory synaptic plasticity.
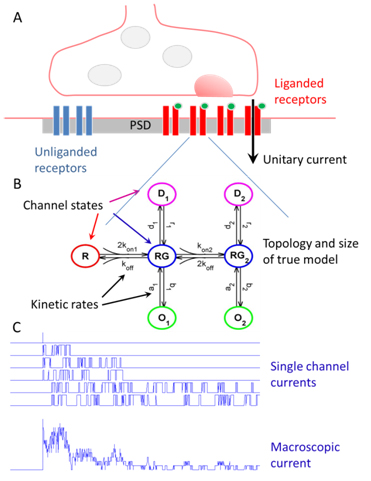
Maximum likelihood estimation of biophysical parameters of synaptic receptors from macroscopic currents
Dendritic integration and neuronal firing patterns strongly depend on biophysical properties of synaptic ligand-gated channels. However, precise estimation of biophysical parameters of these channels in their intrinsic environment is complicated and still unresolved problem. We have recently described novel methods based on a maximum likelihood approach that allows to estimate not only the unitary current of synaptic receptor channels but also their multiple conductance levels, kinetic constants, the number of receptors bound with a neurotransmitter, and the peak open probability from experimentally feasible number of postsynaptic currents (Stepanyuk et al., 2011)(Stepanyuk et al., 2014a). The new method also improves the accuracy of evaluation of unitary current as compared to the peak-scaled non-stationary fluctuation analysis, leading to a possibility to precisely estimate this important parameter from a few postsynaptic currents recorded in steady-state conditions. Estimation of unitary current with this method is robust even if postsynaptic currents are generated by receptors having different kinetic parameters, the case when peak-scaled non-stationary fluctuation analysis is not applicable. Thus, with the new method, routinely recorded postsynaptic currents could be used to study the properties of synaptic receptors in their native biochemical environment.
Further elaboration of this approach that we are doing now will lead to development of user-friendly software that may be widely used to quantitatively study interaction of synaptic channels with pharmacological agents and modulation of channel functioning in normal and pathological conditions.
Peripheral and central mechanisms of neuropathic pain
Role of T-type Ca2+ channels in central processes of nociceptive neurons in maintenance of diabetic neuropathy
Role of T-type Ca2+ channels in central processes of nociceptive neurons in maintenance of diabetic neuropathy Peripheral diabetic neuropathy (PDN), occurring in about 60% - 70% of diabetic patients, is among the most severe consequences of diabetes. Chronic pain characterized by spontaneous deep aching or burning pain, as well as by mechanical allodynia and thermal hyperalgesia is one of the most disturbing symptoms of PDN. However, the specific cellular mechanisms responsible for development and maintenance of peripheral diabetic neuropathy are mainly unknown. We have recently found that different types of nociceptive DRG neurons demonstrate diabetes-induced upregulation of CaV3.2 subtype of T-type Ca2+ channels during different stages of diabetes development (Khomula et al., 2013)(Khomula et al., 2014)(Duzhyy et al., 2015). The upregulation of T-type channels resulted in the increased neuronal excitability of nociceptive neurons revealed by a lower threshold for action potential initiation, prominent afterdepolarizing potentials and burst firing. The upregulation T-type channels and increased excitability of neurons may contribute to thermal hyperalgesia in early diabetes and nonthermal nociception (e.g. mechanical allodynia and hyperalgesia and spontaneous pain) at its later-stage. We hypothesize that T-type Ca2+ channels are functionally expressed in central nociceptive processes and that upregulation of these channels under diabetic conditions amplifies peripheral action potentials in these neurons to busting discharges leading to changes in nociceptive output of lamina I projection neurons of the spinal cord and maintenance of painful diabetic neuropathy. Since direct electrophysiological measurements of T-type channel functioning in central axons of nociceptive DRG neurons are not methodologically feasible we are testing this hypothesis by an innovative combination of state-of-the-art approaches that include a novel whole spinal cord preparation that preserves the complex organization of dorsal roots in the spinal cord, Ca2+ imaging in identified central axons of specific types of primary nociceptive neurons using genetic Ca2+ indicators and profound computer modelling.
Peripheral and central mechanisms underlying inflammatory chronic pain
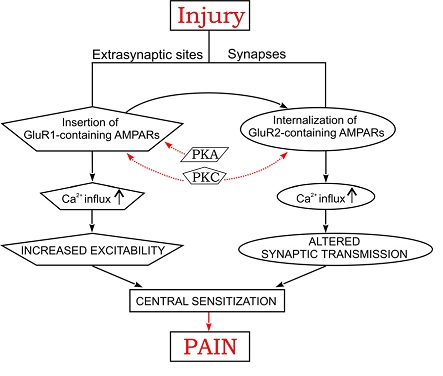
Previous research has resulted in general understanding of importance of Ca2+-dependent glutamate receptor (AMPA receptor subtype) trafficking in the dorsal horn of spinal cord as one of the possible mechanisms of inflammatory chronic pain (Kopach and Voitenko, 2013). It is summarized in Figure 4.
Continuing this research we have recently found that complete Freund adjuvant (CFA)-induced peripheral inflammation, a well-known model of chronic inflammatory pain, prominently augments excitatory neurotransmission in rat dorsal horn lamina II neurons exhibiting adapting firing patterns (Kopach et al., 2015) and apparently representing excitatory glutamatergic interneurons. At the same time this peripheral inflammation decreases excitatory drive to the tonic firing lamina II neurons most of which are inhibitory. The inhibitory drive is also increased to the inhibitory neurons and decreased to the excitatory ones as a result of the inflammation (Kopach et al., 2015). Thus, the balance between excitation and inhibition in the lamina II of dorsal horn is strongly shifted toward the excitation (Kopach et al., 2015). The lamina II interneurons directly synapse onto lamina I projection neurons (PNs), the main output of painful signaling in the spinal cord. Thus, these inflammatory-induced, neuron-type specific changes in synaptic activity in lamina II neurons most probably results in an increase of the excitatory drive and a decrease of the inhibitory drive to lamina I PNs. In its turn it may increase PN excitability, hence contributing to maintenance of the inflammatory pain. In this research we aim to test this hypothesis and to study what cellular and molecular mechanisms underlie increased excitability of PNs under inflammatory conditions.
References
- Borisyuk A, Stepanyuk A, Belan P (2014) Activity-Dependent Potentiation of an Asynchronous Component of GABA-ergic Synaptic Currents in Cultured Hippocampal Neurons. Neurophysiology 46:10–15 [Accessed April 25, 2014].
- Dovgan a V, Cherkas VP, Stepanyuk a R, Fitzgerald DJ, Haynes LP, Tepikin a V, Burgoyne RD, Belan P V (2010) Decoding glutamate receptor activation by the Ca2+ sensor protein hippocalcin in rat hippocampal neurons. Eur J Neurosci 32:347–358 [Accessed March 26, 2014].
- Duzhyy DE, Viatchenko-Karpinski VY, Khomula E V, Voitenko N V, Belan P V (2015) Upregulation of T-type Ca2+ channels in long-term diabetes determines increased excitability of a specific type of capsaicin-insensitive DRG neurons. Mol Pain 11:29 [Accessed August 11, 2015].
- Khomula E V, Borisyuk AL, Viatchenko-Karpinski VY, Briede A, Belan P V, Voitenko N V (2014) Nociceptive neurons differentially express fast and slow T-type ca(2+) currents in different types of diabetic neuropathy. Neural Plast 2014:938235 [Accessed May 7, 2014].
- Khomula E V, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan P V, Voitenko N V (2013) Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim Biophys Acta 1832:636–649 [Accessed March 26, 2014].
- Kopach O, Krotov V, Belan P, Voitenko N (2015) Inflammatory-induced changes in synaptic drive and postsynaptic AMPARs in lamina II dorsal horn neurons are cell-type specific. Pain 156:428–438 [Accessed February 22, 2015].
- Kopach O, Voitenko N (2013) Extrasynaptic AMPA receptors in the dorsal horn: evidence and functional significance. Brain Res Bull 93:47–56 [Accessed October 27, 2015].
- Stepanyuk A, Borisyuk A, Belan P (2014a) Maximum likelihood estimation of biophysical parameters of synaptic receptors from macroscopic currents. Front Cell Neurosci 8:303 [Accessed October 6, 2014].
- Stepanyuk AR, Borisyuk AL, Belan P V (2011) Efficient maximum likelihood estimation of kinetic rate constants from macroscopic currents. Degtyar VE, ed. PLoS One 6:e29731 [Accessed May 8, 2014].
- Stepanyuk AR, Borisyuk AL, Tsugorka TM, Belan P V (2014b) Different pools of postsynaptic GABAA receptors mediate inhibition evoked by low- and high-frequency presynaptic stimulation at hippocampal synapses. Synapse 68:344–354 [Accessed August 22, 2014].
Members
| Name | Work number | ||
|---|---|---|---|
| 1. | Belan P.V. | pasha@biph.kiev.ua | 380442562053 |
| 2. | Kononenko M.I. | ni.kononenko@biph.kiev.ua | 380442562428 |
| 3. | Gryshchenko O.V. | alesha@biph.kiev.ua | 380442562555 |
| 4. | Saftenku О.Е. | esaft@biph.kiev.ua | 380442562085 |
| 5. | Stepanyuk A.R. | standrey@biph.kiev.ua | 380442562499 |
| 6. | Cherkas V.P. | cherkas@biph.kiev.ua | 380442562518 |
| 7. | Dovgan O.V. | rasboinik@mail.ru | 380442562428 |
| 8. | Borisyuk A.L. | borisyuk@biph.kiev.ua | 380442562518 |
| 9. | Krotov V.V. | vovakrotov@gmail.com | 380442562426 |
| 10. | Sheremet E. | sheremetik@yandex.ru | 380442562518 |
| 11. | Dromaretskiy A.V | avi9526@biph.kiev.ua | 380442562518 |
| 12. | Bagatska O.V. | bagatskaya@biph.kiev.ua | 380442562082 |
| 13. | Edutenko M. | yedutenko@gmail.com | 380442562518 |
| 14. | Bozhenko A. | arsebo@biph.kiev.ua | 380442562518 |
| 15. | Burdakova A. | nastia.burdakova@gmail.com | 380442562428 |
| 16. | Osipenko D. | osipenkoden@gmail.com | 380442562428 |
Graduate Biophysics and Physiology
We can offer you graduate training in 2 higher degrees:
- 2 year MSc Programme in Biophysics and Physiology
- 3-4 year PhD Programme in Biophysics and Physiology
For general admission information please refer to:
- Graduate School at Bogomoletz Institute of Physiology
- Teaching & Scientific Centre in Physics & Technology of National Academy of Sciences of Ukraine
Entry Requirements
- Applicants for MSc Programme will be expected to have obtained or to be about to obtain a Honours BSc degree in biophysics, physics or biomedical science from a Ukrainian university, or an overseas degree at an equivalent level.
- Applicants for PhD Programme will be expected to have obtained or to be about to obtain a Honours MSc degree in biophysics, physics or biomedical science from a Ukrainian university, or an overseas degree at an equivalent level. Graduates of our MSc Programme are especially welcomed to apply for and are prevailed in our PhD Programme.
Career Opportunities
Our programmes aim to provide students with a detailed understanding of biophysics, physiology and disciplines related to biomedicine and biomedical equipment. Our graduates leave the Programme with the knowledge and tools deemed essential for both academia and pharmaceutical industry. Our PhD programmes assure getting postdoctoral positions in high-ranking universities worldwide.
Facilities
Laboratory of Molecular Biophysics is equipped with three imaging rigs:
- Two high-speed epifluorescence imaging stations mounted on inverted fluorescent microscopes (Olympus IX-70/71, Olympus, Japan) and monochromator Polychrome V (Till Photonics, Germany), CCD camera SVGA (Imago, Till Photonics, Germany), a computer graphic station and imaging software LA and Till vision (Till Photonics, Germany); one of the setup is combined with a DualView system (Optical Insights, USA) necessary to perform fast FRET recordings. *The stations are equipped with electrophysiological equipment, including two double patch-clamp amplifiers EPC-10/2 (HEKA Elektronik, Germany), patch-pipette puller P-97 (Sutter, USA), two high-precision micromanipulator MP-285 (Sutter, USA) and two hydraulic micromanipulator (Narishige, Japan) and air tables (TMC and Newport, USA).
- Confocal microscope imaging station Fluoview 1000 (Olympus, Japan) having UV, 405, 488, 515, 543 and 633 nm laser lines and mounted on an upright BX61WI microscope (Olympus) (this is shared equipment). It is mounted on an air table and bundled with patch-clamp amplifier Dagan 3900A (Dagan Corporation, USA), AD/DA board (National Instruments, USA), motorized stage (MMTS, Scientifica, UK) and set of micromanipulators (PatchStar, Scientifica, UK), IsoFlex extracellular stimulator (A.M.P.I., Israel) and temperature-controlled perfusion system (Warrner Instruments, USA).
- The Laboratory is also equipped with a fume hood and other laboratory supplies, including a dissection microscope, pH-meter, osmometer, balances, etc. *The laboratory has a real-time qPCR machine, DNA Engine thermal cycler and Nanodrop instrument within 200 feet of lab for common use.
- Tissue Culture Room has two biosafety hoods in space adjacent to his laboratory, as well as 3 cell culture incubators, refrigerators for culture reagents, inverted microscopes and other standard cell culture equipment. The Laboratory has access to shared liquid nitrogen storage for cell lines.