Methods of Department of Cytology
Department of Cytology is primarily interested in studying morphological and functional changes in neural tissue resulted from cerebral ischemia. We particularly focus on quantitative analysis of morphological correlates of neuronal and synaptic plasticity in normal and pathological development.
The Department is equipped with transmitted light and electron microscopes (Jeol 1010 and Selmi TEM 100M), and has sufficient facilities for cell and slice culturing and in vivo experiments. The contacts is highly experienced in different types of microscopy, including ultrastructural analysis, histochemistry, immunohistochemistry (including gold-immunolabeling), computer-assisted morphometry.
The Department pioneered neural tissue culturing in Ukraine, and we have good experience of using various tests for estimating the cell culture viability.
- Light and electron microscopy
- Immunohistochemistry
- Cultures of cells and slices
- Models of ischemic damage
- Assays to estimate cell viability
LIGHT AND ELECTRON MICROSCOPY
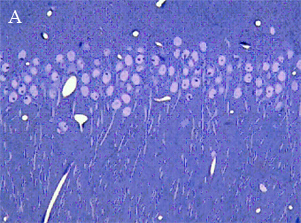
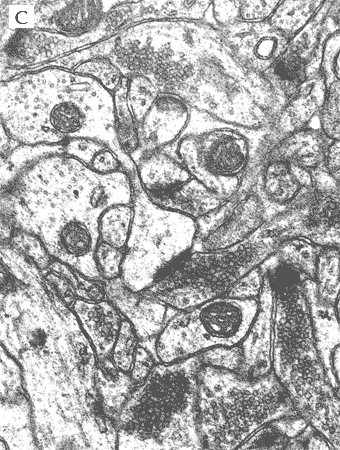
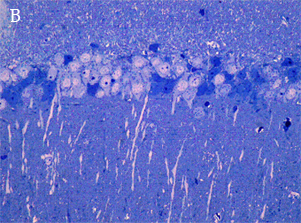
In the most of our experiments, light (A and B) and electron (C) microscopy followed by morphometric analysis is used to determine the altered properties of neural and glial cells on histological and ultrastructural levels. This includes regular and quantitative microscopic analysis as well as identification of cells and cell compartments using immunolabelling.
|
|
|
back to top
IMMUNOHISTOCHEMISTRY
Light microscopy immunohistochemistry This is a very powerful tool in neurocytology for the demonstration of the presence of proteins in cells of a tissue section. We use indirect method of immunostaining. This method involves an unlabeled primary antibody which reacts with tissue antigen, and a labeled secondary antibody which reacts with the primary antibody. The second antibody can be labeled with an enzyme (A) or a fluorescent dye (B). The latter method is of great use in confocal laser scanning microscopy, which is highly sensitive and can also be used to visualise interactions between multiple proteins (C).
Triple staining vizualized with Confocal Imaging System FV1000-BX61WI (Olympus, Japan). Cellular nuclei (A), neurons (B) and astrocytes (C) are identified by labelling with (Hoechst 33258 (blue), anti-NeuN (green) and anti-GFAP (red) in separate and merged (D) view.
Changes in rat brain during early stages of diabetes mellitus development revealed with double staining for neuronal (NeuN) and astroglial (GFAP) marker.
A - control, B - 7 days from diabetes onset.
back to top Electron microscopy immunohistochemistry Immunocytochemictry approach allows to have an impression of labeled proteins localization at the ultrastuctural level. Using HRP reaction (black reaction) we showed GFAP-positively stained astrocytes profiles (A). Astrocyte process localizes appose to blood vessel (BV). Within dark process there are mitochondria (mit) and synaptic terminal (st) with pre- and post-synaptic structures. Next approach to visualize proteins localization at the electron microscopic level is immunolabelling by gold particles (B). Astrocyte process was labeled with nano gold particles (1.4nm) followed by silver enhancement technique.
back to top CULTURES OF THE CELL AND SLICES Hippocampal dissociated cell cultures The most important benefit of dissociated cell cultures is the possibility to control precisely the environment affecting cells, to access easily and continuously individual neurons for morphological and electrophysiological analyses, pharmacological manipulations, and high-resolution microscopic monitoring at the level of a single cell and synaptic terminal. Hippocampal organotypic slice cultures The organotypic cultures, in addition to the advantages of dissociated cultures, keep preserved during weeks of culturing the natural morphology, different cell types, local neuronal layers and connections, synaptic organization, the time-course of synaptic development, etc. The slice cultures represent a system, which is more close to in vivo conditions, and is to be used for further development of neuroprotective tools. Endothelial dissociated cell cultures Endothelial cells of cerebral vessels growing as a monolayer are used to quantitatively estimate alterations in nitric oxide (NO) metabolites (Gris reaction) and to analyze morphologically the effects of various damaging agents (e.g., irradiation) on the endothelial ultrastructure. back to top MODELS OF ISCHEMIC DAMAGE Studying ischemic damage to the brain While the integrated mechanisms of ischemia and the effects of drug interventions are readily studied in in vivo models, the complexity of such models does not permit detailed studies of particular molecular mechanisms and cellular events. These limitations are overcome in the in vitro models of ischemic damage where the contribution of blood components are eliminated and parameters of extracellular environment can be standardised and controlled. In vivo models: In adult rats, four-vessel-occlusion (4VO) followed by normoxic reperfusion is used to initiate events occurring in the brain after global cerebral ischemia. Two-vessel-occlusion (2VO) is applied to another rodents, gerbils, which do not have arterial circle of Willis. In vitro models: Oxygen-glucose deprivation (OGD) is used in vitro to simulate cell damage and cell death resulted from ischemic conditions in the brain. The cultures are placed into an air-tight chamber with temperature control. For an ischemic episode, which may last for minutes and hours, the cell medium is rapidly replaced by glucose-free medium and air is rapidly replaced by oxygen-free gas mixture. This may be followed by returning the cell cultures to normoxic conditions for hours and days (reoxygenation). The resulted cell damage is usually delayed and depends on duration of both the episode of deprivation and the period of reoxygenation. OGD may be applied to both dissociated and organotypic cultures. The response of the hippocampal cultures to lesions induced by ischemic conditions has been shown to be similar to that shown by animals in the situation in vivo suggesting the suitability of the cultures for the study of ischemic lesions and neuroprotective drugs. back to top ASSAYS TO ESTIMATE CELL VIABILITY Diferent methods are used to estimate cell viability and metabolic activity: Propidium Iodide (PI) staining is the commonly used approach for visualizing cell degeneration and estimating cell viability. PI is a stable dye entering dying cells with damaged cell membrane. Once inside a cell, PI interacts with DNA to yield a brightly red fluorescence. PI is basically non-toxic to neurons and is used as a marker of neuronal membrane integrity and cell damage.
OGD + 1 h OGD + 24 h Trypan blue is another non-toxic vital dye which also enter cells with damaged membrane and stain their nuclei in blue (the result is visible in regular light). Tetrazolium-Formazan Assays measure redox activity of viable cells and are based on estimating the bioreduction of soluble tetrazolium salt (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide, MTT, MTS, other salts) to insoluble (if using MTT) or soluble (if using MTS) formazan by metabolically active cells (presumably, by mitichondrial and cytosolic enzymes). The amount of formazan, to be measured colorimetrically, is proportional to the number of living cells and is widely used for evaluating cell viability in culture. LDH Efflux Assay. Lactate dehydrogenase (LDH) is a stable cytosolic enzyme catalyzing the last reaction of glycolisis. When the plasma membrane is damaged, it is rapidly released outside the cell, and its efflux is to be measured colorimetrically in the cell culture supernatant to be used as a reliable biochemical index for cell membrane damage. back to top
